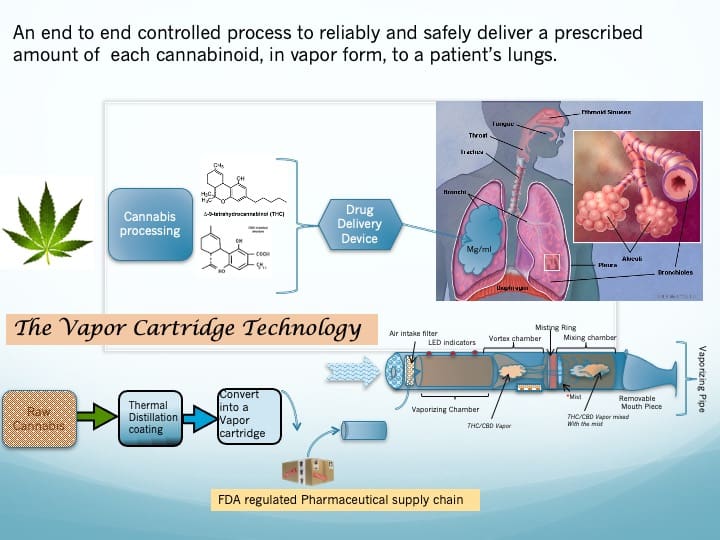
The Science
A Technical Presentation of the Vapor Cartridge Technology
Succinctly, the Vapor Cartridge Technology is an inexpensive, environmentally friendly way to efficiently extract, a precise amount of pure cannabinoids and terpenes from botanical cannabis (a.k.a. Flower) and deposit those extracts within to a unique (and patented) VCT vapor cartridge. The VCT vapor cartridge packaging can meet the requirements of the FDA for distribution through either the liquor channels or pharmaceutical channels. The consumer inserts the VCT vapor cartridge into a unique (and patented) drug delivery device which will deliver a pure precise dose of cannabis extracts in vapor form.
Once successfully commercialize, Vapor Cartridge Technology products will dramatically change the cannabis industry; VCT vapor cartridges will, overtime, replace flour and vape pens as the vehicle to produce and inhale vapors that are rich in cannabis extracts. This customer base is enormous and rapidly growing. Furthermore, the market will grow exponentially once the USA and Mexico join Canada and legalize cannabis for medical and adult recreational use.
Technical Overview:
The elegance of this technology is in its straightforward use of basic physics. Fact: Hot vapors condense on cold surfaces. A real-life example is steam from a shower condenses on a bathroom mirror creating a thin coating of liquid water. In the Thermal Distillation Coating (TDC) process hot air is driven through a screened container of finely ground botanical cannabis resulting in a hot vapor that is rich in cannabis extracts. These vapors condense onto a very cold surface of a thin sheet of aluminum within the coating chamber. That sheet can be removed from the coating chamber. When a current is driven through the conductive sheet it heats up rapidly, like the wires in a toaster. The coating is volatized back into the vapor that was previously coated onto the sheet. The technical details on how to produce a VCT vapor cartridge and how to design the associated drug delivery device is intended for the licensee’s engineering team assigned to commercialize the technology. The business and marketing team can skip over these technical details and go directly to the Impact Statement of this seminal technology.

The Vapor Cartridge Technology consists of three system components:
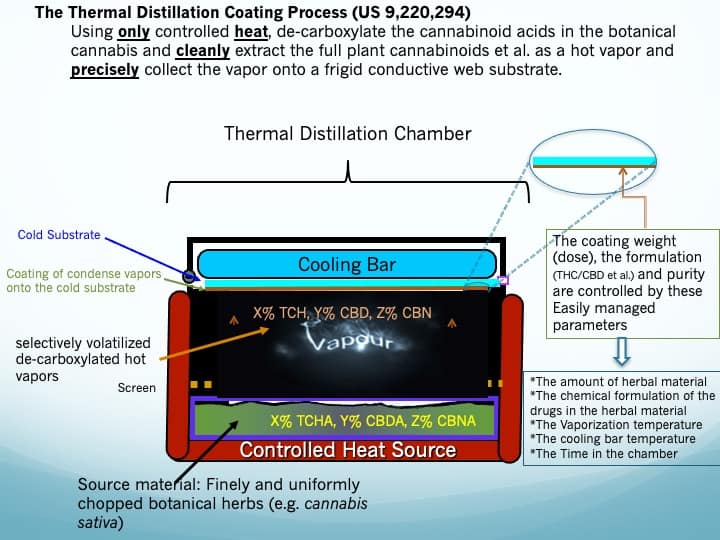
#1: Thermal Distillation Process (US 9,220,294 et al.)
This process utilizes the physical properties of the acidic cannabinoids contained within the botanical cannabis. The acidic cannabinoids can be decarboxylated and vaporized at a much lower temperature than the temperature at which the toxins in the plant material volatize resulting in a toxin free, hot vapor that is rich in neutralized cannabinoids. This is a ‘whole plant’ extraction in that it extracts all of the 150 plus cannabinoids, all terpenes as well as trace flavonoids, carotenoids and chlorophylls. Thus, the specific varietal of botanical cannabis controls the formulation.
Referring to figure 1, the pure hot cannabinoid vapors et al., contained within the thermal distillation chamber, condense onto a chilled conductive substrate, coating it densely with cannabinoids et al. This process can be well controlled.
The coating weight (measured in gm/cm2) and its formulation (i.e., , percentage, by weight, of each cannabinoid and terpene) are a function of easily measured and controlled process parameters. One parameter is the strain of cannabis used in the coating process as this defines the formulation.
The thermal distillation process produces a sheet of conductive substrate with a precise, highly concentrated, coating of cannabis extracts.
#2. The method for converting the coated sheet substrate into a Vapor Cartridge (US 9,408,986 et al.)
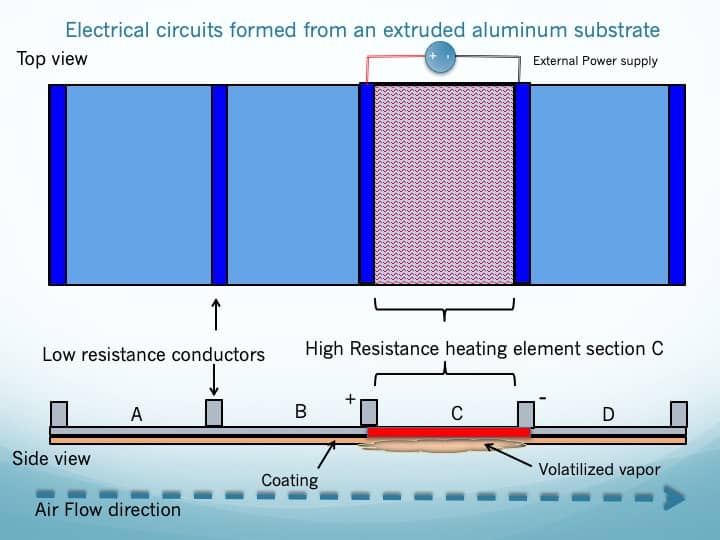
- The Vapor Cartridge is constructed from an extruded aluminum substrate. Liquid aluminum is extruded to create a conductive substrate which consists of multiple parallel electrical circuits defined by parallel, low-resistance, conductors on either side of high-resistance heating elements (please refer to figure 2 to understand the basic concept of volatilizing the coating on the substrate). When a current is driven through one of the parallel conductors through the high resistance heating element section returning to the other parallel conductor, that element heats up and volatilizes the coating of cannabinoids previously deposited.
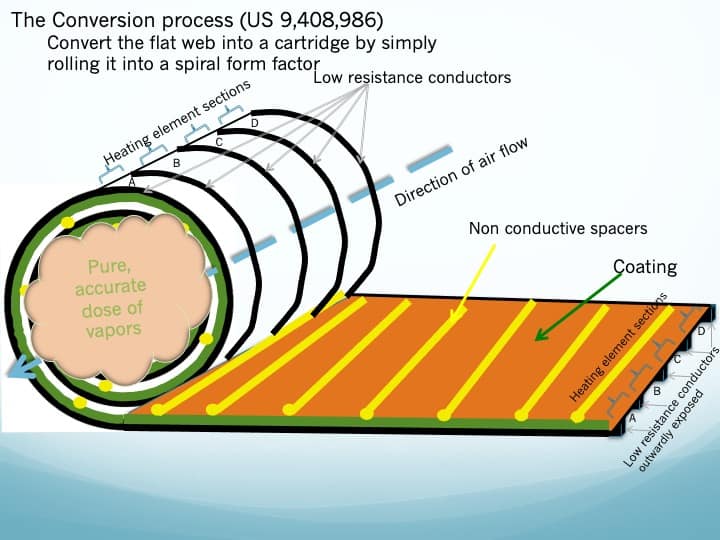
- Referring to figure 3, the process to converting the flat extruded aluminum substrate, coated with its high concentration of cannabinoids, and into a Vapor Cartridge is to simply roll it up into a spiral with the multiple parallel conductors outwardly exposed. Non-conductive spacers create an open airflow tunnel through the Vapor Cartridge.
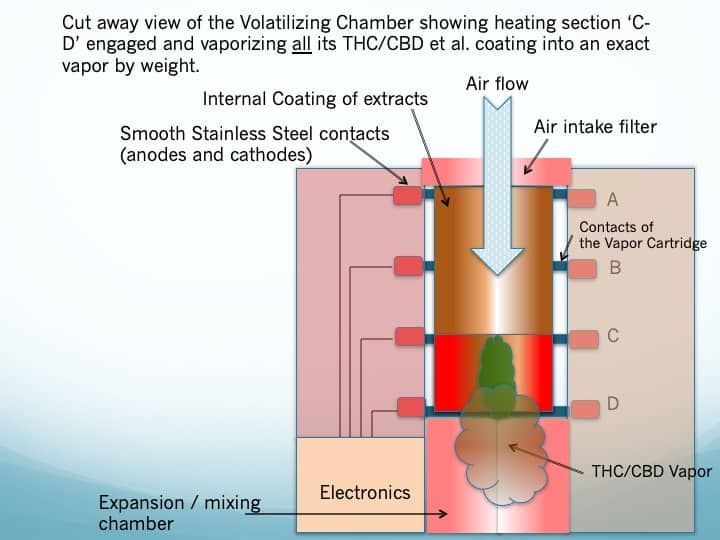
- The volatilizing chamber of the Drug Delivery Device performs the critical function of extracting the cannabinoids. Figure 4 shows a functional block diagram depicting how the exposed parallel conductors of the Vapor Cartridge match up to the contacts in the volatizing chamber. When directed by the patient, a well-controlled current is driven through the selected section, volatizing its coating of cannabinoids.
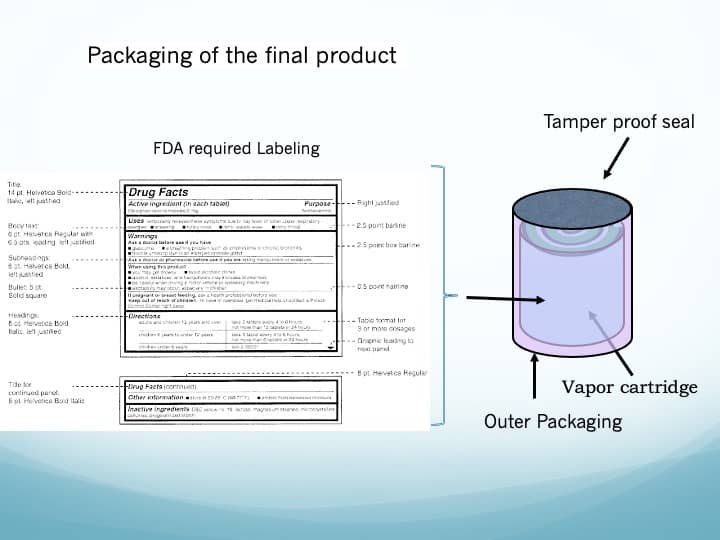
- Referring to figure 5, the ability of the Vapor Cartridge form factor to meet all local, state, and federal packaging, labeling, and tracking requirements is of enormous value. This would enable the Vapor Cartridge to get approved for distribution through both the vast pharmaceutical channels and the distribution channels for adult beverages!
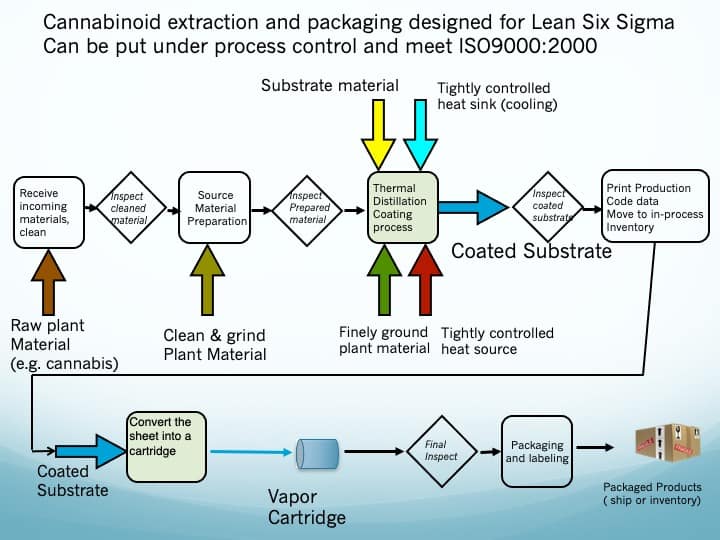
- The Thermal Distillation Process is also a very environmentally friendly process.
- No chemicals are used
- The spent organic material can be put back into the soil
- The aluminum Vapor Cartridge is recyclable
- The Vapor Cartridges themselves have a long storage life, allowing processors to use finished product inventory to balance changing seasonal supply with changing demand.
#3: The Drug Delivery Device (US 9,380,813 et al.)
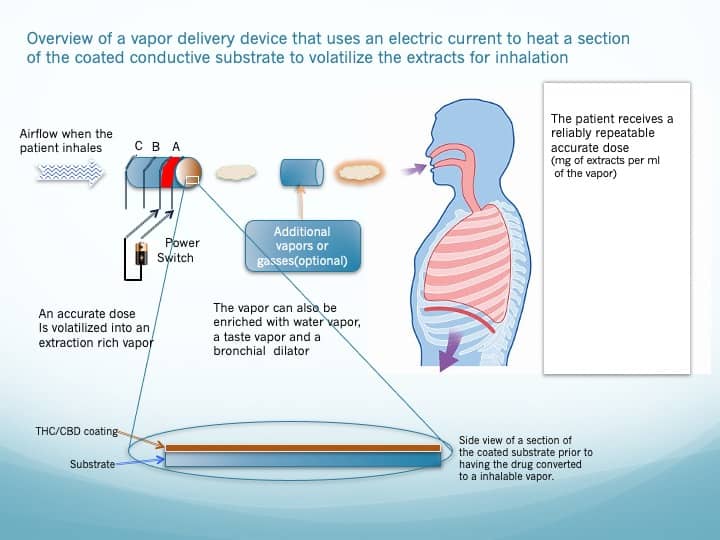
Figure 6 depicts the basic concept of extracting the cannabinoids, from the Vapor Cartridge by driving a current through two exposed parallel low-resistance conductors of a given section, heating that section, and vaporizing the drugs therein. A significant advantage of this design is that the cannabinoid-rich vapors can be mixed with other beneficial vapors, for example:
Water vapor would moisturize the air passage
A taste vapor (e.g., menthol) would make the process more pleasant
A bronchial dilator would improve the drugs’ transfer into the bloodstream.
- Figure 7 depicts a physical Drug Delivery Device designed to implement the concepts conceptually described in figure 6. (A detailed technical explanation will be given to prospective licensee(s))
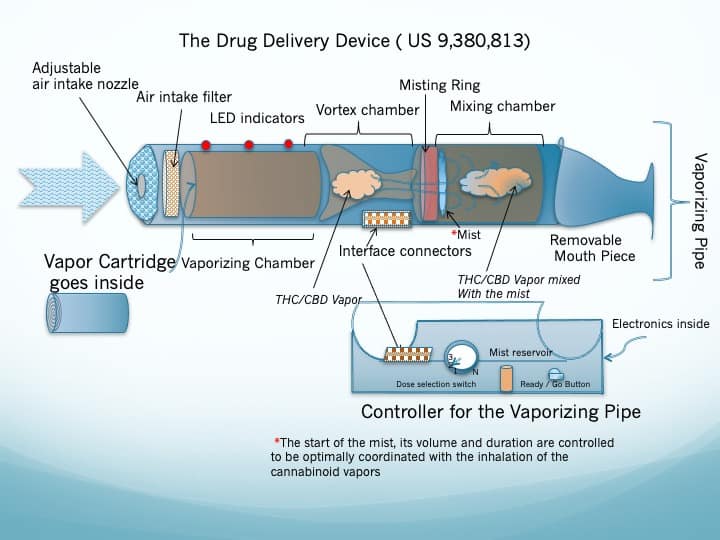
Figure 8 graphically depicts the essence of this fantastic technology. The function of the technology, shown in the top view, is to extract the cannabinoids et al. from raw botanical cannabis and deliver the drugs safely and accurately to the patient’s lungs. The process for doing that is shown in the bottom view and each step in this process has been detailed in the previous pages.
DRUG DELIVERY SYTEM WITH STACKABLE SUBSTRATES
STACKABLE SUBSTRATES IN A REUSABLE CARTRIDGE:
- The spiral design allows for a large surface area to accommodate a large number of drugs. Furthermore, continuous roll feed coating can result in high-volume low-cost manufacturing. However, handling and converting a thin roll of material into a precise spiral is a challenging undertaking that not many manufacturers do. And, those special manufacturers that are skilled at handling web converting (e.g. 3M, SWM, Bemis, Tesa) are not companies that may prioritize entering into the drug delivery market.
- In order to broaden the category of manufacturers that could successively commercialize the Vapor Cartridge Technology we had to greatly simplify the manufacturing process. After years of research, we invented and patented a process that creates the required surface area, not by rolling up a film strip, but rather by stacking several sheets of flat-coated substrates. This alternative design of the Vapor Cartridge would be to stack flat coated substrates that can be inserted into a reusable cartridge and such substrates would have been selectively coated using a mask such that portions of the substrate are not coated and thus can be electrically conductive. The mask is a permanent part of the thermal distillation chamber and is made from non-electrically conductive thermally stable material such as ceramics. Furthermore, the stackable flat substrates here can have sufficient strength and firmness for stacking and easy handling and requires a very simple converting process. Time to market can be significantly shortened and yields can be very high.
Figure 9 shows an overview of the process for forming and transporting the selectively coated substrate.
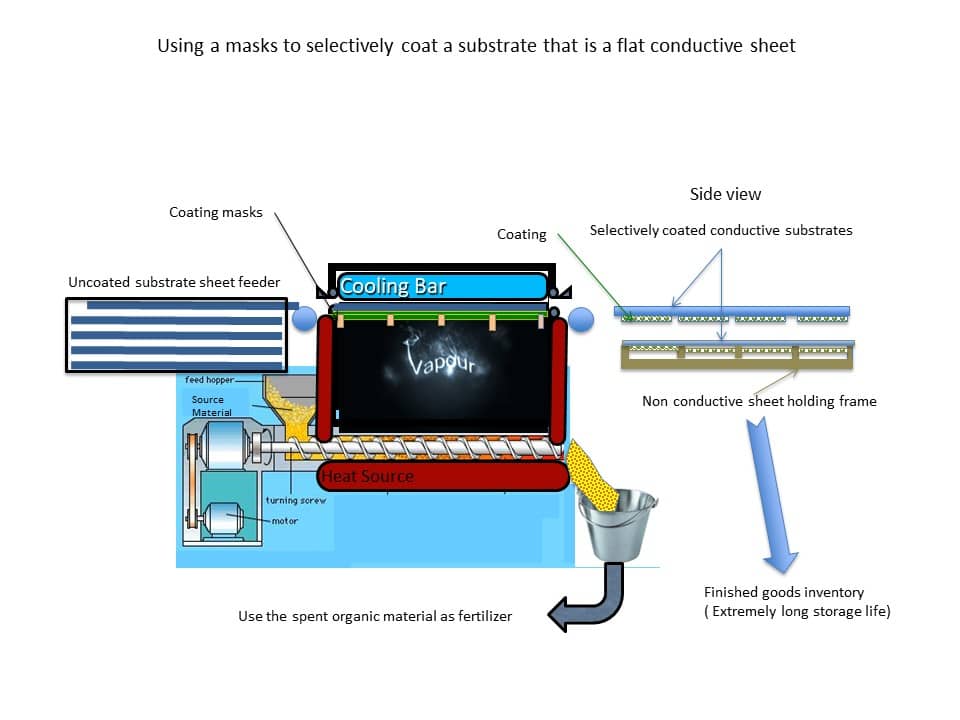
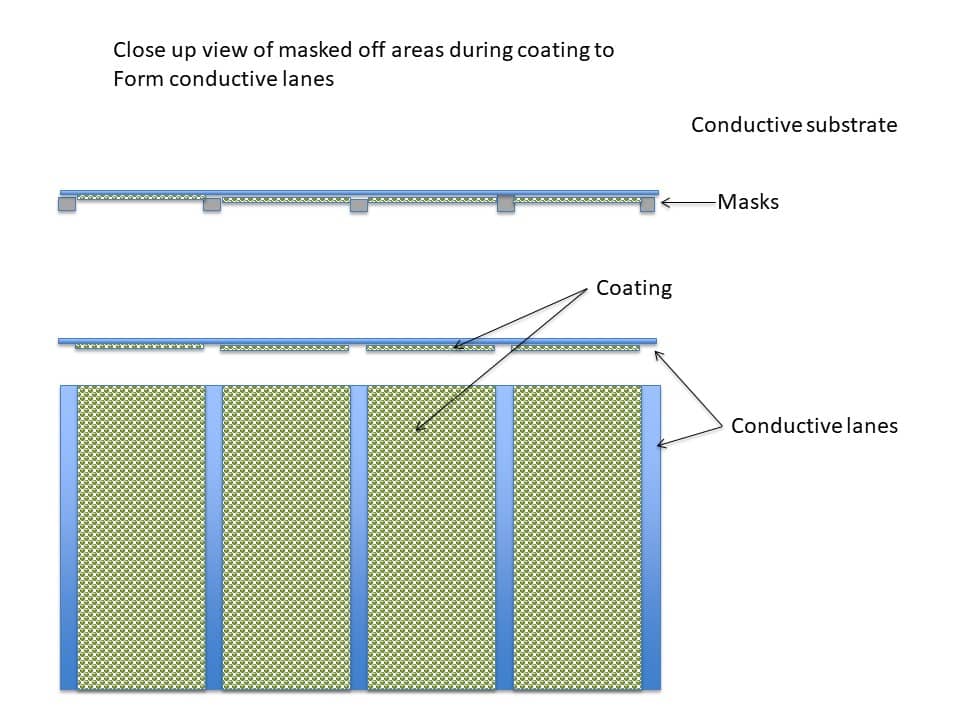
Figure 10 below shows additional views of the substrate to further illustrate masked off areas during coating.
Next, the selectively coated substrate can be loaded onto a non-conductive holding frame, as shown in Figure 11 below.
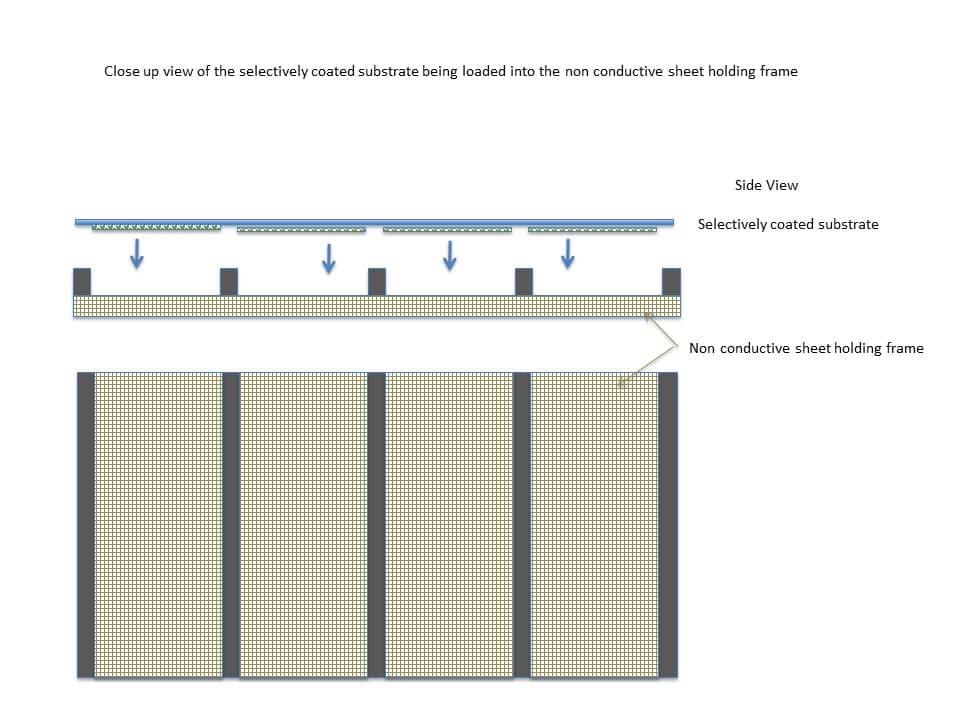
The holding frame can be formed from a non-conductive material capable of supporting the selectively coated substrate. In an example, the holding frame can be cardboard. The holding frame can include protrusions that align with the uncoated portions of the coated substrate.
Individual sheet holding frames can be stacked for packaging, as shown in Figure 12 below.
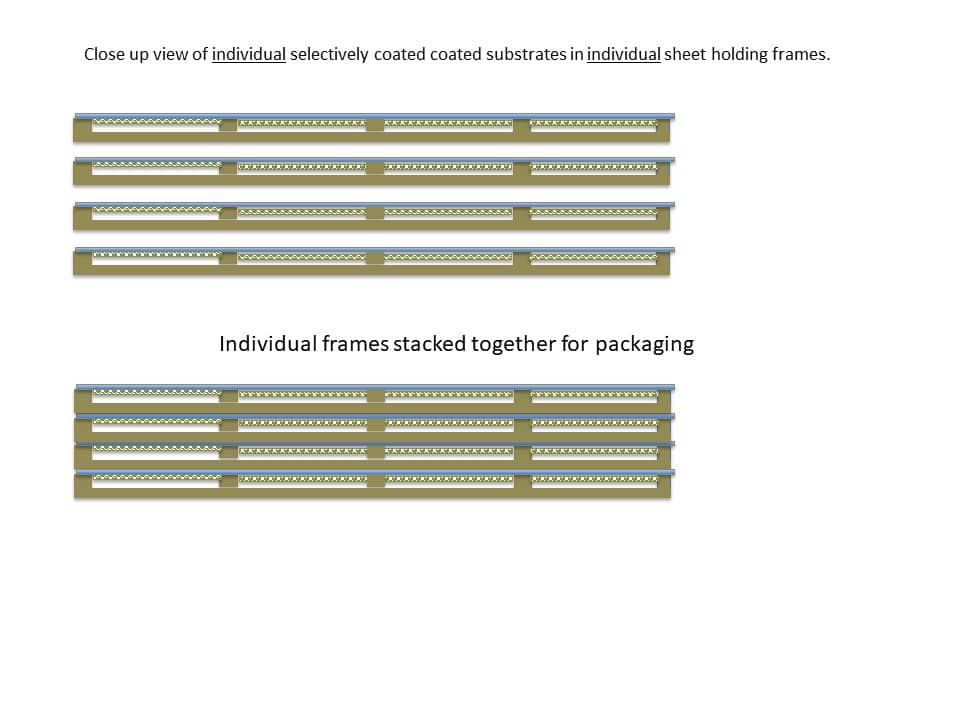
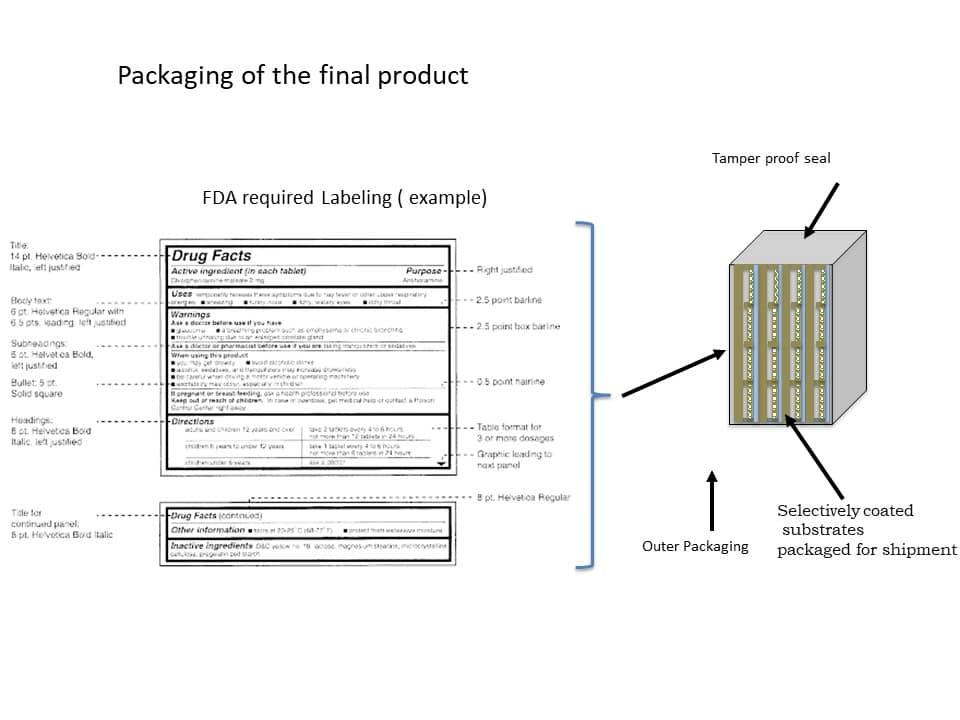
Figure 13 below shows an example of a packaged product ready for shipment. This is described further below.
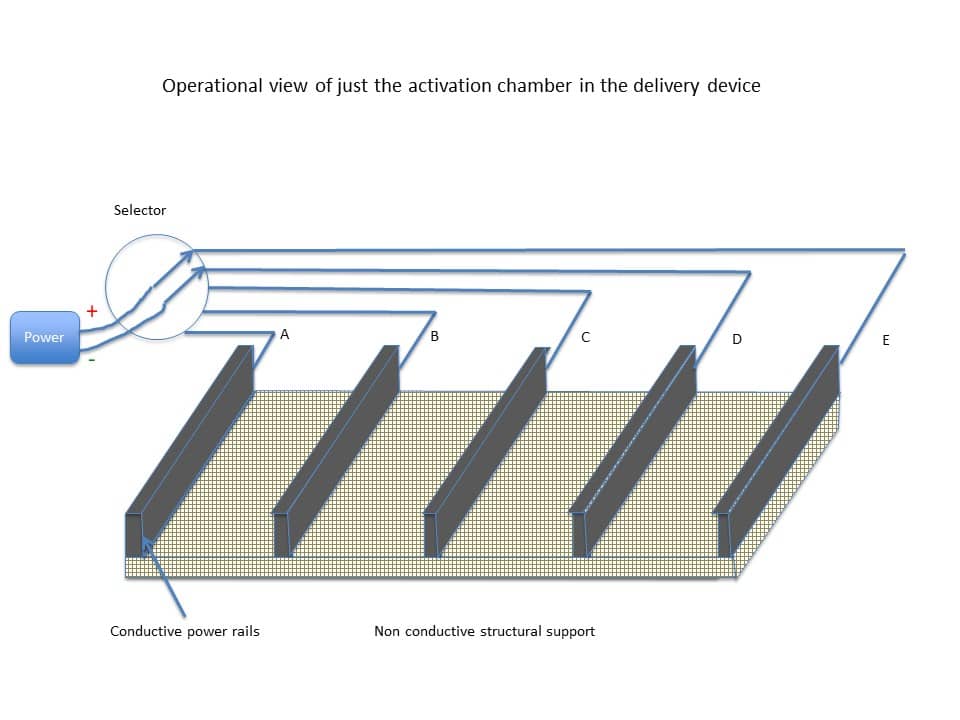
The drug delivery device can be configured to receive one or more selectively coated substrates. The drug delivery device can include a cartridge chamber that serves as an activation chamber for the substrate. The cartridge or activation chamber (in the drug delivery device) is shown in Figure 14 below.
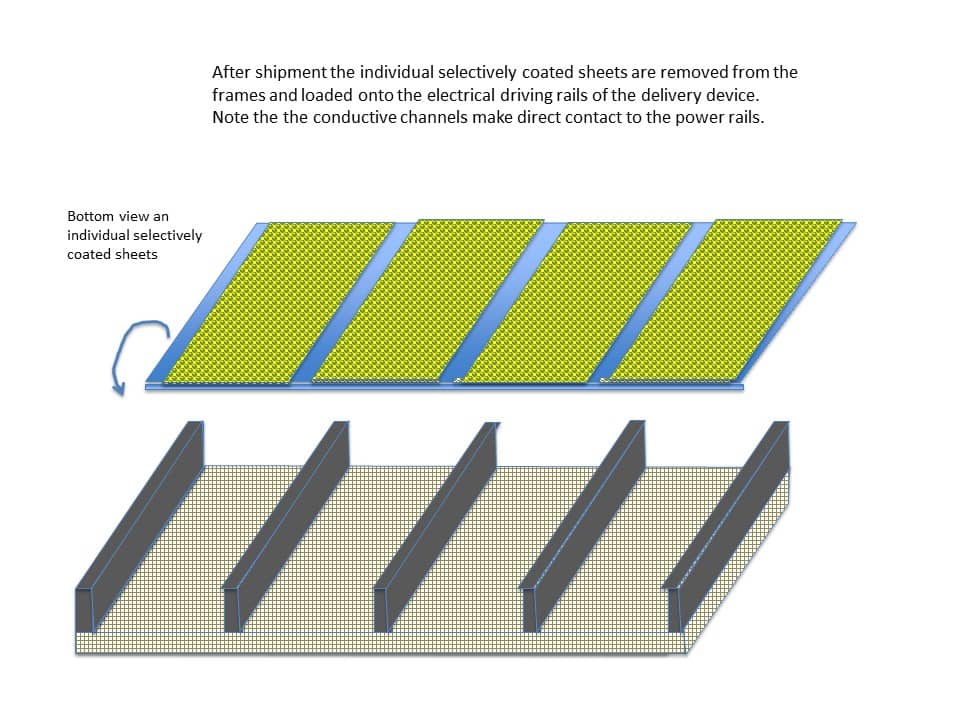
Upon receiving the package of selectively coated sheets, each sheet can be removed from the frame and loaded onto the power rails in the cartridge forming an activation chamber. Note that the conductive channels (the uncoated portions of the substrate) can make direct contact with the power rails as shown in Figure 15.
Once the sheet is secure inside the activation chamber, one or more sections of the chamber can be electrified through the power rails driving an electrical current through the conductive substrate. This heats one or more sections of the substrate and causes the coating on that particular section or sections of the substrate to volatilize into a vapor. Figure 16 shows an example activation chamber having four sections (A-B, B-C, C-D, and D-E) where section D-E is being electrified.
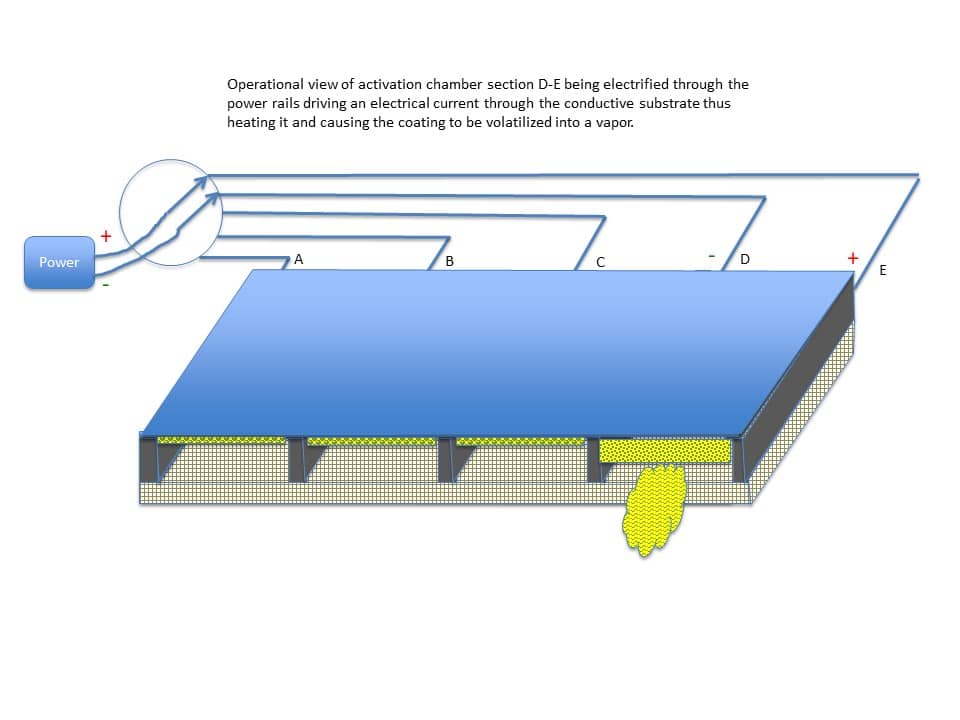
In other examples, more or less sections can be included in the design of the activation chamber. Similarly, the activation chamber can be designed to receive multiple sheets of the selectively coated substrate. In an example shown in Figure 17, three substrates can be stacked inside the activation chamber.
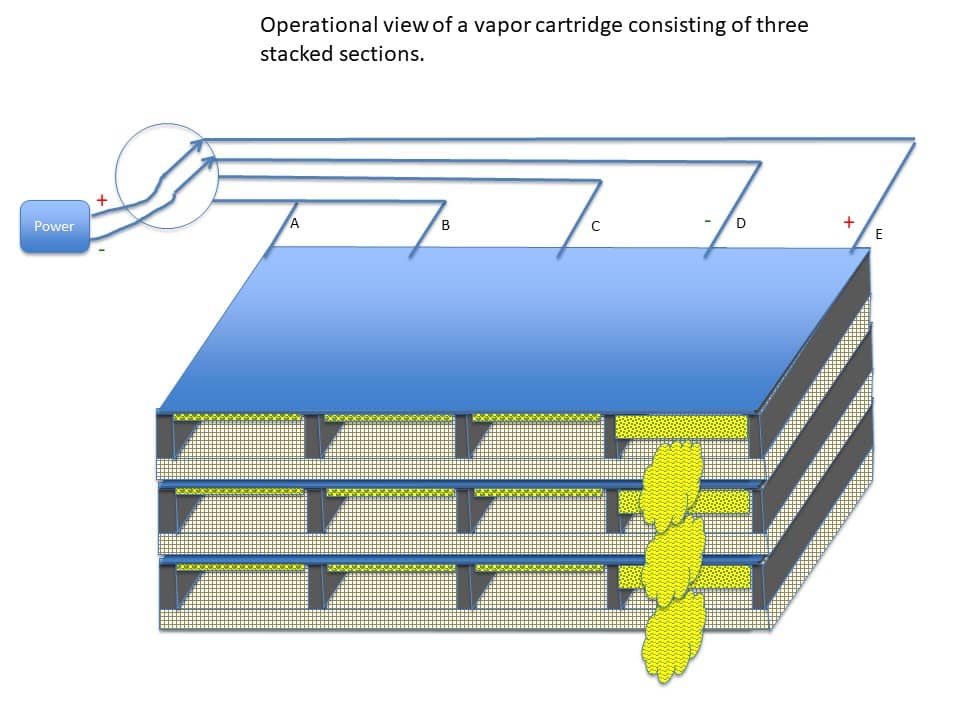
Figure 18 shows the vapor cartridge secured inside the drug delivery device.
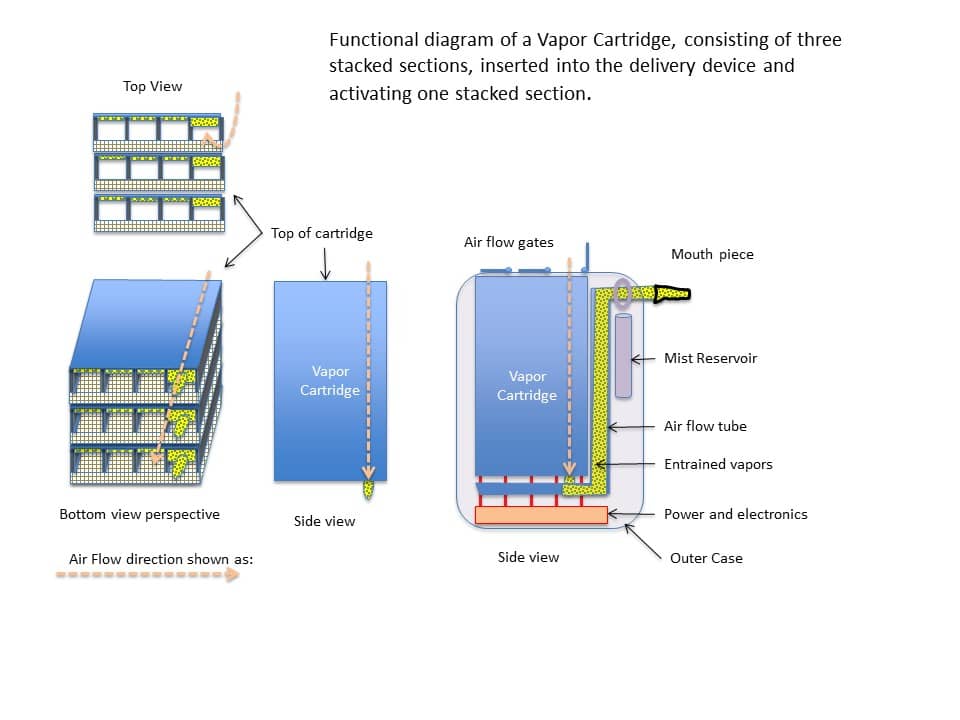
After the drug on the three coated substrates has been volatilized, the vapor cartridge can be removed from the drug delivery device and the individual substrate sheets can be removed from the vapor cartridge. The sheets can be disposed of (recycled) and the new sheets can then be inserted into the vapor cartridge. As such, the vapor cartridge (which serves as a housing for the sheets) can be reusable.
In some instances, it may be desirable to replace one of the three sheets without replacing all three sheets. The design of the vapor cartridge makes this feasible.
Impact Statement:
Once successfully commercialized the Vapor Cartridge Technology will be a game changing, paradigm shifting, disruptive technology!
- It will be, far and away, the most cost-efficient manner to deliver cannabinoid vapors as measured by $/mg/ml of cannabinoids in the bloodstream. Even when heavily taxed, the process will still produce a very profitable product because it will be in high demand (Exceeding customers’ expectations) and still be the least expensive recreational product at the retail level, well below black market ‘street price’. From a customer’s perspective, it’s “A better high at a lower price”.
- It will be the only technology that can provide the dose control, formulation control, and purity assurance of inhaled botanical vapors required of an FDA-approved drug.
- This is the rapid-onset, non-smoked cannabinoid delivery system that the Institute of Medicine (IOM) has long been requesting.
- It defines a Drug Delivery Device that will meet ISO 11.040.01 and thus can be approved by the FDA for the delivery of ingestible cannabinoid vapors. This will quickly become the medical and recreational standard for ingesting cannabinoid drugs in vapor form.
- It defines a Vapor Cartridge manufacturing process that is highly efficient, high capacity, and high quality (meeting ISO 9001:2000). Furthermore, the process was designed with controllable inputs and measurable outputs enabling state-of-the-art statistical process control and six-sigma discipline to drive continuous improvement for years to come (such as yield, capacity, throughput, unit cost, and overall quality). Furthermore, the commercialization process will lead to additional intellectual properties in terms of patents, trade secrets, and trademarks.
The Vapor Cartridge construction enables it to meet all the logistical and regulatory requirements of the TTB, ATF, and state-regulated distribution channels for alcoholic beverages for adult recreational use. Thus, providing profits for all in the supply chain (farmers, processors, distributors, and retailers). Furthermore, this supply chain has the enormous benefit of being able to use the existing physical and communication infrastructure which can easily and efficiently regulate and collect taxes. Furthermore, retail sales through wine and liquor stores provide both convenience for the customer and the assurance of no sales to minors.
- It will significantly increase the value of legal cannabis crops as source material for vapor cartridge processing while substantially devaluing, even eliminating, the retail demand for raw botanical cannabis (a.k.a. dried flower heads) as a means for extracting and ingesting cannabinoid vapors. Note: Not all raw cannabis is illegal, but all illegal cannabis is raw.

